Worldwide, coal remains one of the largest sources for energy, contributing to approximately one fourth of the global energy supply and over one third of the fuel that is used to produce electricity. [1] It has been projected that coal will be used to produce approximately 40% of the electricity worldwide and 34% in the United States until 2040. [2]
Coal dust particles are created during the process of coal production. Excessive exposures to coal dust can overwhelm the lungs’ mechanism to clear these particulates, causing them to accumulate over time. [3] Because of modern technological advancements, significant volumes of coal can be mined per shift. [4] Through constant exposure and inhalation of coal dust particles, coal miners are at an increased risk for developing respiratory illnesses categorized as coal mine dust lung disease (CMDLD). These pulmonary conditions can range from airflow limitation or obstruction to causing interstitial lung diseases.
When these particles are introduced into the respiratory tract, they can cause a reactive process in the lung tissue known as pneumoconiosis. [5] Coal workers’ pneumoconiosis (CWP) is also known as “black lung disease,” one of the most common conditions that belong in the category of CMDLD, along with silicosis, mixed-dust pneumoconiosis with coexistent silica exposure, chronic bronchitis, emphysema, and dust-related diffuse fibrosis. [1, 4]
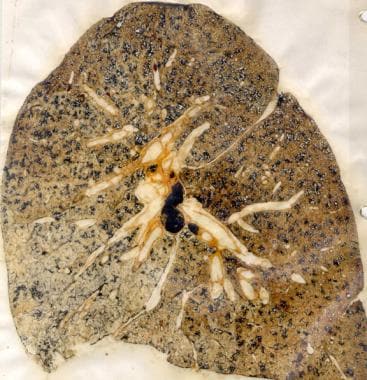
CWP is divided into two categories: simple CWP (SCWP) and complicated CWP (CCWP), or progressive massive fibrosis (PMF), [3, 4] depending on the extent and severity of the disease. Also see Silicosis and Coal Worker Pneumoconiosis. Note the images below.

--> Coal workers' pneumoconiosis (black lung disease). Gross specimen demonstrating simple coal worker's pneumoconiosis.
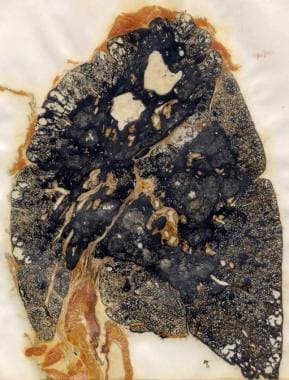
--> Coal workers' pneumoconiosis (black lung disease). Gross specimen demonstrating progressive massive fibrosis in a coal miner.
No cure for CWP currently exists. Treatment for both SCWP and CCWP is symptomatic, and prevention is the key to management of this condition. Lung transplantation has been used in the setting of end-stage pulmonary disease.
Anthracosis has previously been used synonymously for coal workers’ pneumoconiosis (CWP) (black lung disease) or for describing the process of detecting a substantial amount of pulmonary carbon deposits on autopsies secondary to recurrent exposure to several factors, such as air pollution, smoke inhalation, or coal dust fragments. [6, 7] Dust particles as small as five microns can enter the lungs and infiltrate the peripheral bronchioles and alveoli. The presence of these particles can obstruct the airways and lead to primary lesions composed of coal dust, macrophages, and fibroblasts. [5] Accumulation of these particulate matter in the lung tissues can be the stimulus to the development of several pathologic conditions described as harmless pulmonary anthracosis, emphysema, and/or lung fibrosis. [4]
The exact mechanism for how pneumoconiosis develops is still unclear; however, several theories have been proposed to help explain its pathogenesis, including oxidative stress theory, immunology, cytokine network theory, and single nucleotide polymorphisms. [8] Mossman described that lung tissue as a common organ affected by inhaled pollutants such as metals, mineral dust, particulates, and reactive gases. [9] Vanhee and coworkers reported three phenomena that occur after the pulmonary tissues are exposed to coal dust: (1) Inflammatory cells accumulate and activate inflammatory cells in the lower respiratory tract; (2) fibroblast proliferation; and (3) augmented formation of extracellular matrix components. [10] It has also been suggested that macrophages play a significant role in the development of pneumoconiosis. [8, 9, 10, 11, 12, 13] Zhang and colleagues indicated that the presence of dust causes the alveolar macrophages to induce an immune response that may ultimately lead to fibrosis. [8]
The oxidative stress theory is one of the possible mechanisms to help describe the pathogenesis of pneumoconiosis. This theory suggests that exposure to dust particulates in the lung tissues provokes an inflammatory response, which then leads to the formation of reactive oxygen and nitrogen species (ROS/RNS) by alveolar and interstitial macrophages, along with polymorphonuclear cells. [9] Mossman describes alveolar and interstitial macrophages as playing a role in oxidative stress through an oxidative burst response that produces ROS/RNS. However, other cells, such as epithelial cells, can also generate a similar reaction. [9] Therefore, the reactive process by macrophages and epithelial cells may be the stimulus for an immune response and functional changes that occur in surrounding cells, including fibroblasts, which can lead to the development of fibrosis. In addition, Pinho and coworkers described the production of ROS leading to other changes in the lungs, such as inactivation of antiproteases, disturbances in the basal membrane, and injury to the alveolar cells. [11]
Another possible mechanism to explain the occurrence of pneumoconiosis is the production of cytokines. Besides the production of ROS, [10, 12, 13, 14] macrophages are also thought to release mediators (eicosanoid metabolites, destructive proteolytic enzymes, and inflammatory growth and differentiation factors), [10] and cytokines have been implicated in the development of pneumoconiosis. These cytokines have been described as signaling proteins that influence inflammatory and fibrotic reactions. Several proinflammatory cytokines have been identified with the development of this condition including interleukin (IL)-6, tumor necrosis factor-α (TNF-α), and IL-1β. [14]
An investigative study by Ulker and colleagues evaluated the serum and bronchoalveolar lavage (BAL) inflammatory cytokine levels in a total of 85 subjects with simple pneumoconiosis (SP) and progressive massive fibrosis (PMF). [12] Active and retired miners served as control subjects. The study results demonstrated that subjects with SP and PMF had higher levels of IL-1β, IL-6, and TNF-α in serum and BAL. [12]
Single nucleotide polymorphisms (SNPs) are another suggested mechanism for the pathogenesis of CWP, entailing the notion that certain individuals may be at increased risk for developing CWP because of their genetics. There have been discussions regarding the concept of allelic variations with SNPs being more frequently identified, and that there are certain functional variants that may be conducive to potentiating an increased risk for certain disease states. [15, 16] Previous studies investigating an association between gene polymorphisms involving TNFα and CWP in Belgian and Japanese coal miners demonstrated that those with CWP had a higher rate of TNFα-308 variant compared to those without the lung disease. [16] In a case control study, Wang and coworkers evaluated the association between three functional SNPs in the SELE gene (T1880C/rs5335, T1559C/rs5368, and A16089G/rs4786) with the risk for developing CWP in the Han Chinese population. [15] Their results demonstrated that there may be an association between SELE rs5368 and an increased risk for CWP. [15]